
Hybridoma screening platform
Hybridoma technology is an important monoclonal antibody technology. Based on cell fusion technology, it fuses B lymphocytes from immunized animals with myeloma cells. Through HAT selection and specific screening, and subcloning, monoclonal antibody cell lines that can specifically recognize the target antigen are obtained. The cell lines are cultured in vivo or in vitro, and monoclonal antibodies are obtained after affinity purification.
Hybridoma technology is an important monoclonal antibody technology. Based on cell fusion technology, it fuses B lymphocytes from immunized animals with myeloma cells. Through HAT selection and specific screening, and subcloning, monoclonal antibody cell lines that can specifically recognize the target antigen are obtained. The cell lines are cultured in vivo or in vitro, and monoclonal antibodies are obtained after affinity purification.
Preparation Process
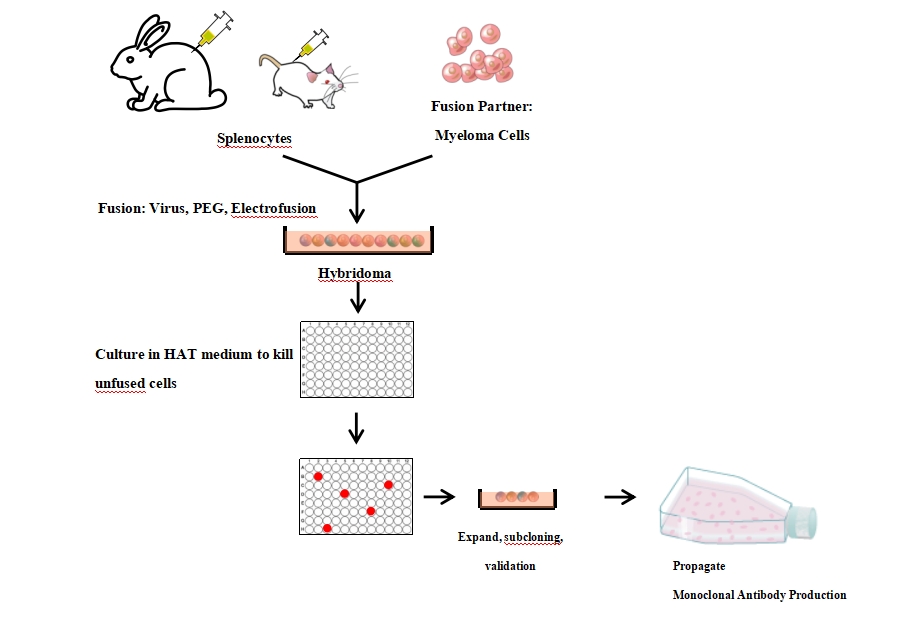
Preparation Principle
- B cells can secrete and express antibodies; one B cell clone produces one antibody.
- B cells are primary cells and cannot be passaged indefinitely in vitro.
Myeloma cells are permanently passaged tumor cells, also derived from B cells, but do not secrete antibodies themselves. Myeloma HGPRT enzyme is deficient and cannot survive in HAT medium.
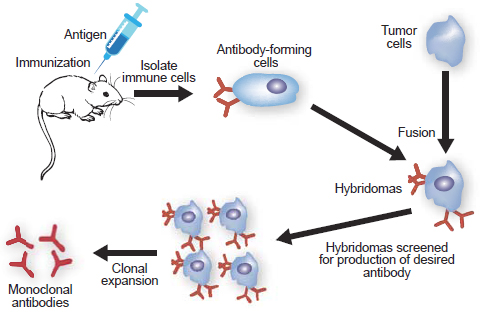
Service Content
Hybridoma Screening
Service Items | Content | Cycle | Delivery |
Antigen Preparation | Antigen design and expression verification, large-scale expression | 4 - 8 weeks | Antigen design and verification report, express antigen according to needs |
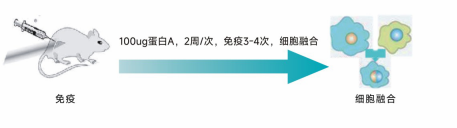
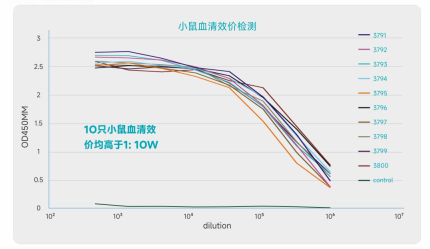
Animal Immunization | Antigen activity identification (optional), mouse immunization, titer detection | 8-10 weeks | Mouse immunization plan, titer detection report |
Fusion, Screening and Subcloning | Cell fusion, fusion detection, subcloning and cryopreservation | 6 - 8 weeks | Antibody fusion and subclone detection report, cryopreserved cell lines |
Antibody Production, Purification and Activity Identification | Antibody serum-free culture, antibody purification, antibody activity detection | 2 - 4 weeks | Purified antibody, activity detection report |
Hybridoma Cell Serum-Free Culture and Domestication Technology
Service Items | Content | Cycle | Delivery |
Serum-free culture and purification of antibodies | Serum-free culture of antibodies, antibody purification and purity detection, antibody activity detection (optional) | 2 - 4 weeks | Purified antibody, antibody purity detection report, antibody activity detection report (optional) |
Hybridoma Cell Serum-Free Domestication | Direct domestication of hybridoma cells, gradient domestication of hybridoma cells, serum-free expression and purification of domesticated cells (optional) | 4 - 8 weeks | Cell domestication report, cryopreserved cells (domesticated), purified antibody (optional) |
Hybridoma Antibody Sequencing Technology
Service Items | Content | Cycle | Delivery |
Hybridoma Cell Sequencing | Hybridoma total RNA extraction, 5'RACE cDNA preparation, antibody VH/VL gene amplification, TA cloning and sequencing, result analysis | 1 week | Sequencing report, antibody sequence, vector containing antibody sequence |
Service Cases
Immunization Plan
Titer Detection
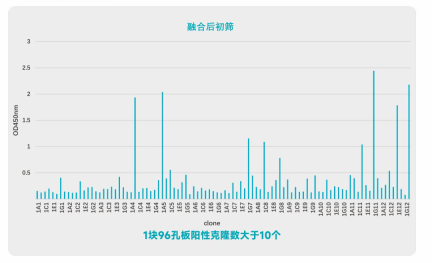
Fusion Detection
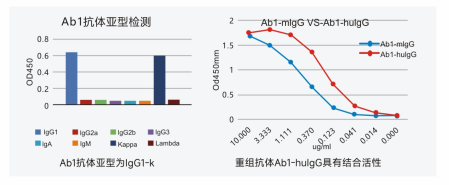
Antibody subtype & Recombinant antibody expression identification
Documents


