
Fully human Fab synthetic library
Constream Biotech currently has a library capacity of 10 11 of a fully human Fab synthetic library. The ultimate goal is to establish a 100-billion-level fully human Fab synthetic library, dedicated to developing high-affinity, high-specificity candidate antibodies for pharmaceutical companies and diagnostic reagent companies.
Constream Biotech currently has a library capacity of 10 11 of a fully human Fab synthetic library. The ultimate goal is to establish a 100-billion-level fully human Fab synthetic library, dedicated to developing high-affinity, high-specificity candidate antibodies for pharmaceutical companies and diagnostic reagent companies.
Service Advantages
● Optimal germline sequences: suitable for phage display, high expression, and good druggability
● Randomization strategy: Trimer primers, diverse CDR3 lengths, and diverse amino acid distribution
● Optimal library capacity: Library capacity close to 10 11 Good diversity, reduced ineffective screening
● Widest application: Verified by dozens of targets, suitable for different types of targets, affinity reaching pM
Service Content
Serial Number | Main Steps | Content | Cycle | Delivery Results |
1 | Antigen Preparation | Customer provides 2mg antigen for screening and preliminary identification |
|
|
2 | Phage Library Screening (Fully Human Fab Synthetic Library) | 1. 3-4 rounds of panning 3. ELISA ranking | 4-8 weeks | Up to 40 sequences will be provided in batches (20 in the first batch, 20 in the second batch) for full antibody expression and activity verification by the customer. |
3 | Full Antibody Expression and Purification | The antibodies obtained from phage screening will be converted into full antibody forms (IgG1 or customer-specified full antibody forms) for expression and affinity purification. | 2-3 weeks | Up to 1mg protein per sequence (concentration >1mg/ml, 1-step purification: SEC >95%, SDS-PAGE >95%) |
4 | Total Cycle | 6-11 weeks | ||
Service Cases
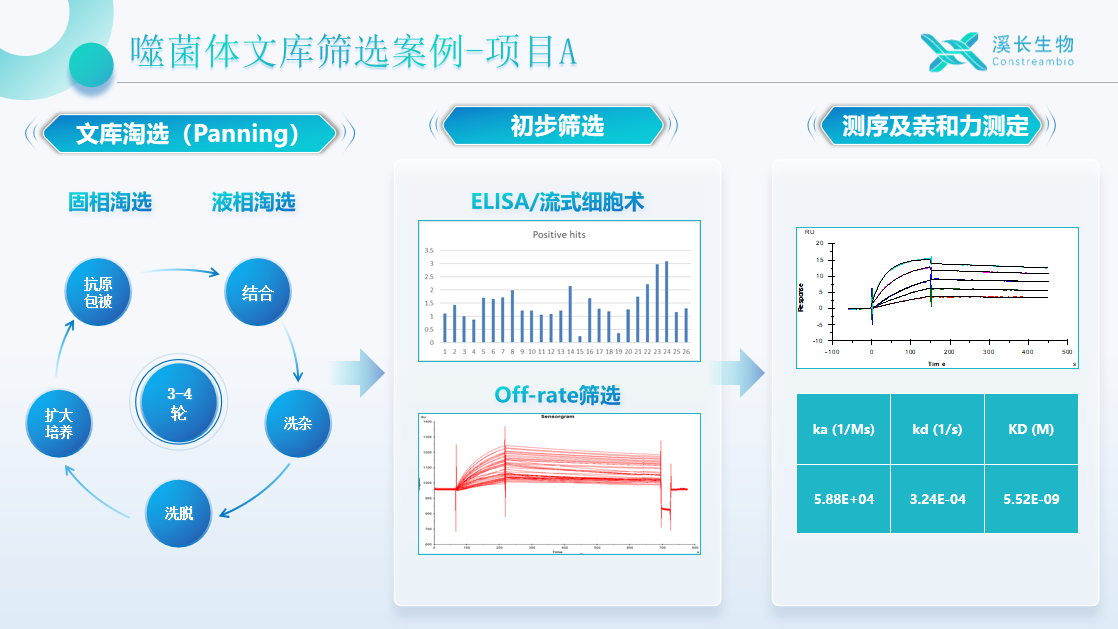
Synthetic Phage Library Display Technology Screening Cases
Documents


