
Nanobody discovery platform
Llama-derived nanobodies (VHH antibodies or single-domain antibodies) are single-domain antibodies isolated from the naturally occurring heavy-chain antibodies (HCAbs) found in camelids (such as llamas and camels). With a molecular weight of only approximately 15 kDa, they are the smallest functional antigen-binding units currently known. Their unique structure and biological properties offer immense potential in the fields of biomedicine, diagnostics, and industry.
Llama nanobodies (VHH antibodies or single-domain antibodies) are single-domain antibodies isolated from heavy-chain antibodies (HCAbs) naturally occurring in camelids (such as llamas and camels). With a molecular weight of only about 15 kDa, they are the smallest functional antigen-binding units currently known. Their unique structure and biological characteristics have shown great potential in biomedicine, diagnostics, and industry.
Compared with traditional antibodies, they have the following core advantages:
1. Ultra-small size and high permeability
Molecular weight of only 15 kDa: much smaller than traditional IgG (150 kDa) and scFv (25-30 kDa), it can penetrate solid tumors, the blood-brain barrier, or dense tissues, suitable for tumor targeting and treatment of central nervous system diseases.
No Fc dependence: avoids binding to traditional Fc receptors, reducing non-specific effects (such as cytokine release syndrome).
2. High stability and resistance to extreme environments
High temperature resistance, acid and alkali resistance: VHH antibodies can maintain their activity at high temperatures (>80°C), extreme pH (pH 2-12), or high concentrations of denaturants (such as urea), suitable for oral administration or industrial enzyme immobilization.
Long shelf life: can be stored at 4°C or room temperature for a long time, reducing cold chain transportation costs.
3. High affinity and specificity
Binding to cryptic epitopes: The CDR3 region of VHH is longer and more flexible, and can recognize concave or cleft epitopes (such as hidden sites of viral spike proteins) that are difficult for traditional antibodies to access.
Low cross-reactivity: Precise screening through phage display reduces binding to non-target molecules.
4. Short development cycle and low cost
No humanization required: Llama VHH has high homology with human VH (>80%), and can be directly used in humans, avoiding the humanization process of murine antibodies.
High-efficiency screening platform: Phage library screening can obtain candidate molecules within 2-3 months, significantly faster than traditional hybridoma technology (6-12 months).
5. Multifunctional engineering potential
Modular design: can be flexibly constructed into multivalent antibodies, antibody-drug conjugates (ADCs), CAR-T targeting structures, etc.
Fusion tags: Add His tags, fluorescent proteins, or radioisotopes to adapt to diagnostic and imaging needs (such as PET-CT tracing).
Service Process
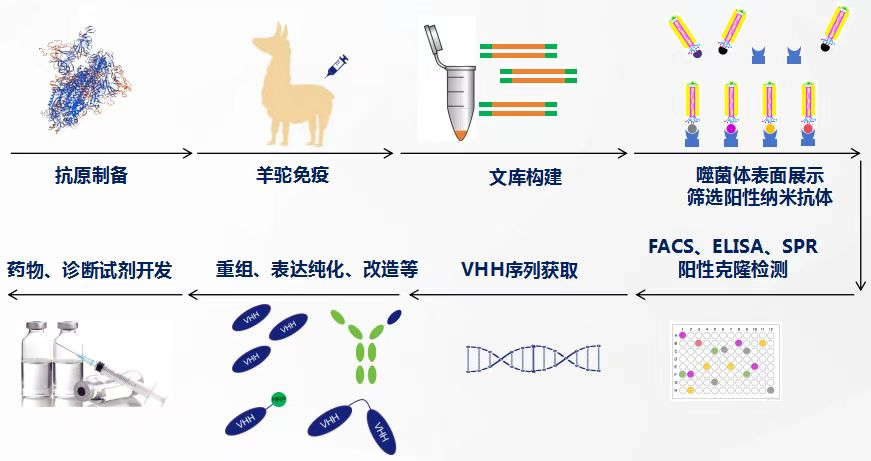
Service Content
Serial Number | Main Steps | Content | Cycle | Delivery Result |
1 | Antigen Preparation | 1. Customer provides 3-5mg antigen expressing his, Fc-tagged antigen (optional) | 2-3 weeks | Obtain sufficient antigen for immunization and identification |
2 | Animal Immunization | Immunize 1-2 llamas/immunogen | 8-10 weeks | Post-immunization titer greater than 1:100000. If the serum is less than 1:5000, booster immunization or termination of the experiment can be chosen. |
After 4 immunizations, llama serum is collected for antigen ELISA detection. | ||||
Phage Display Nanobody Library Construction and Screening | 1. Extract mononuclear cell RNA and reverse transcribe it into cDNA, nested PCR amplification of nanobody gene; | 4-6 weeks | Provide at least 10 nanobody sequences that specifically bind to the antigen | |
1. Select screening scheme according to antigen characteristics | ||||
3 | Nanobody Expression and Purification | 1. Perform prokaryotic or eukaryotic expression of nanobodies according to customer requirements | 2-3 weeks | Provide >0.5mg purified antibody |
4 | Humanization (optional) | Sequence analysis | 2-3 weeks | Humanized sequences and protein samples (1mg/antibody) |
5 | Document Writing and Transfer | Complete and transfer antibody materials and sequence file reports | 1 week | Transfer relevant technical report documents (vector sequencing report, purified protein verification report) |
6 | Total Cycle | 20-24 weeks | ||
Documents


