05
2025
-
01
Xi News | Antibodies to Watch in 2025: 11 domestically developed antibody products have applied for listing
The commercial development of antibody therapeutics is a global endeavor involving tens of thousands of biopharmaceutical companies and supporting service organizations. Their combined efforts have resulted in over 200 antibody therapeutics on the market to date, with nearly 1400 more product candidates in various stages of clinical trials for a wide range of diseases.
The commercial development of antibody therapeutics is a global endeavor involving thousands of biopharmaceutical companies and supporting service organizations. To date, their combined efforts have brought more than 200 antibody therapeutics to market, and nearly 1400 product candidates in various stages of development are undergoing evaluation in clinical trials for the treatment of diverse diseases. Here, we discuss key events in antibody therapeutics development in 2024 and predict key events likely associated with the late-stage clinical development pipeline in 2025. Specifically, we report on 21 antibody therapeutics that received their first approval in at least one country or region in 2024, including the bispecific antibodies tarlatamab (IMDELLTRA®), zanidatamab (Ziihera®), zenocutuzumab (BIZENGRI®), odronextamab (Ordspono®), ivonescimab (依达方®), and the antibody-drug conjugate (ADC) sacituzumab tirumotecan (佳泰莱®). We also discuss 30 investigational antibody therapeutics with marketing applications under review by at least one regulatory agency as of our last update. December 9, 2024, including ADC drugs datopotamab deruxtecan, telisotuzumab vedotin, patritumab deruxtecan, trastuzumab botidotin, becotatug vedotin, and trastuzumab rezetecan. Among the 178 antibody therapeutics in late-stage development that we included, we summarize key data for 18 that may submit marketing applications by the end of 2025, such as the bispecific or multispecific antibodies denecimig, sonelokimab, erfonrilimab, and anbenitamab. Key trends in the development and approval of antibody formats such as bispecific antibodies and ADCs are reported, along with the clinical-stage transition and global approval success rates of these antibody molecular forms.
We analyzed trends and success rates for different antibody molecular formats and categorized the molecules based on the following criteria:
1) Specificity (i.e., monospecific or bispecific/multispecific);
2) Conjugation status (i.e., antibody-drug conjugates (ADCs, here limited to antibodies conjugated to cytotoxic drugs), radioimmunotherapies (including radioimmunoconjugates (RICs) and antibody chelator conjugates), and immunoconjugates (i.e., antibody conjugates not included in the ADC or radioimmunotherapy groups consisting of antibodies fused to non-immunoglobulin-derived protein domains);
3) Composition (i.e., a single molecule or a mixture). In our analysis, each molecule is represented only once, categorized according to the extent of alteration of the molecule's properties due to modifications. Thus, the ADC group includes bispecific ADCs, the radioimmunotherapy group includes bispecific ADC radioimmunotherapies, and the immunoconjugate group includes immunoconjugate mixtures.
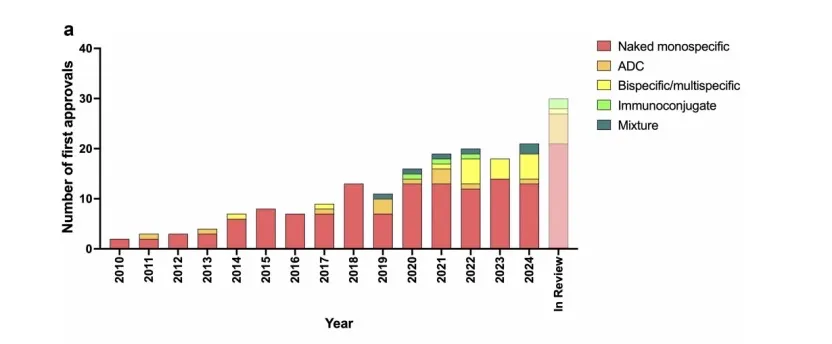
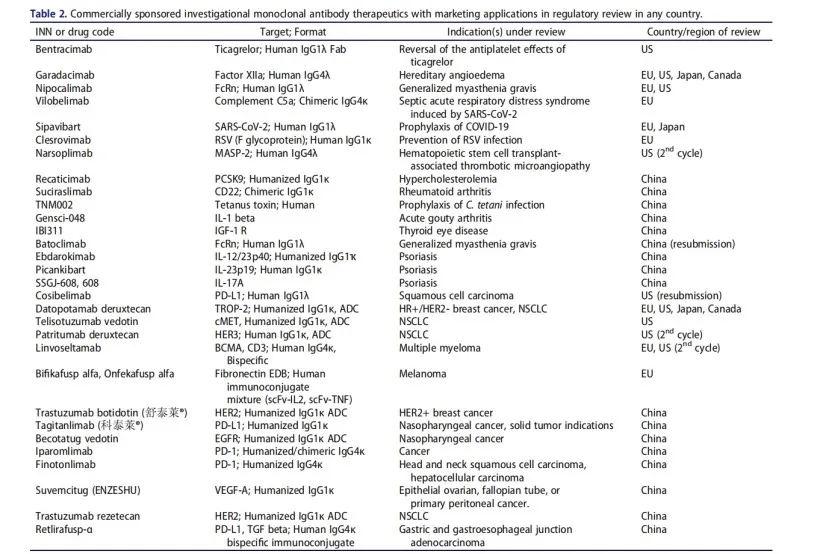
We identified 30 investigational (i.e., not approved for marketing in any country) antibody therapeutics whose marketing applications, including BLAs submitted in the US, MAAs submitted in the EU, and NDAs submitted in China, are under review by at least one regulatory agency as of our last update on December 9, 2024 (Table 2). Thirteen of these applications are under review in the US or China, the EU, and other possible countries, while 17 are under review only in China. Sixteen applications are for non-cancer indications, and 14 are for cancer indications. Details of these molecules are summarized in Table 2, in order.
In 2024, 11 antibody products were approved for marketing by domestic independently developed enterprises, as follows:
Xingmeng Biopharmaceutical (Suzhou) Co., Ltd.'s independently developed and produced Class 1 innovative drug, Zemeiloweimazuo Ruiwei monoclonal antibody injection (Krebi®), an anti-rabies cocktail monoclonal antibody.
Hengrui Medicine's subsidiary, Suzhou Shengdiya Biopharmaceutical Co., Ltd.'s independently developed Class 1 new drug, IL-17A inhibitor, Funachi Zhu monoclonal antibody injection, was launched for the treatment of adult patients with moderate to severe plaque psoriasis suitable for systemic or phototherapy.
Chongqing Zhixiang Jinta Biopharmaceutical Co., Ltd.'s independently developed Class I new drug, recombinant fully human anti-IL-17 monoclonal antibody, Sailichi monoclonal antibody injection (Jinlixi®), for the treatment of moderate to severe plaque psoriasis.
Connoa Biopharmaceutical Technology (Chengdu) Co., Ltd.'s independently developed Class 1 new drug, IL-4Rα antibody drug, Kanyueda (Sipuchibai monoclonal antibody injection), was approved for marketing by the National Medical Products Administration (NMPA) for the treatment of chronic rhinosinusitis with nasal polyps.
Yixining® (Inusi monoclonal antibody injection) is a PCSK9 monoclonal antibody new drug independently developed and innovated by Zhongshan Kangfang Biopharmaceutical Co., Ltd., which was approved for marketing in September 2024, mainly for the treatment of primary hypercholesterolemia and mixed hyperlipidemia.
The marketing authorization application for Ongorexis monoclonal antibody injection (recombinant humanized anti-PCSK9 monoclonal antibody injection, trade name: Junshida®), independently developed by Shanghai Junshi Biosciences Co., Ltd., was approved for the treatment of primary hypercholesterolemia (non-familial) and mixed dyslipidemia in adult patients.
Shiyao Group's therapeutic biological product Class 1 new drug, recombinant anti-PD-1 fully human monoclonal antibody, Enlangsubai monoclonal antibody injection (trade name: Enshuxing®), was approved for marketing for the treatment of patients with PD-L1-positive recurrent or metastatic cervical cancer that has failed at least one line of platinum-based chemotherapy.
Qilu Pharmaceutical's Apalolituowo Reili monoclonal antibody injection (Qibeian®) was approved for marketing by the National Medical Products Administration for the treatment of recurrent or metastatic cervical cancer. Apalolituowo Reili monoclonal antibody injection is the world's first approved PD-1/CTLA-4 dual-functional combination antibody, composed of IgG4 type PD-1 antibody and IgG1 type CTLA-4 antibody in a specific ratio.
Zhengda Tianqing Pharmaceutical Group's Class 1 innovative drug, Bemosu monoclonal antibody injection Benmelstobart (TQB2450) (trade name "Andwei"), was approved for marketing by the National Medical Products Administration (NMPA) for its first indication, in combination with anlotinib hydrochloride capsules, carboplatin, and etoposide for first-line treatment of patients with extensive-stage small cell lung cancer (ES-SCLC). Bemosu monoclonal antibody injection is a novel humanized anti-programmed death ligand-1 (PD-L1) monoclonal antibody.
Idafeng® is a globally innovative PD-1/VEGF bispecific tumor immunotherapy independently developed by CSPC Pharmaceutical. It is indicated in combination with chemotherapy for the treatment of EGFR-mutated locally advanced or metastatic non-squamous non-small cell lung cancer (nsq-NSCLC) that has progressed after treatment with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs). Idafeng® is the world's first approved bispecific antibody drug with a synergistic anti-tumor mechanism of "tumor immunity + anti-angiogenesis."
Sichuan Kelun Biotech's first independently researched TROP2 ADC, sacituzumab govitecan (sac-TMT, Jiatailai®), has been submitted for approval for use in adult patients with unresectable locally advanced or metastatic triple-negative breast cancer who have received at least two prior systemic therapies (at least one of which was for advanced or metastatic disease).
Information source: Antibodies to watch in 2025 https://doi.org/10.1080/19420862.2024.2443538
Keywords:
Reference Drug | Antibody Drug | Instrument | Reagent



