27
2025
-
03
Seizing the Opportunity: SOX17, the Next PD-1?
The immune system is the body's core defense against tumors, but cancer cells often camouflage themselves through "immune escape" mechanisms to avoid detection and attack by immune cells. Currently, immune checkpoint therapy, represented by PD-1/PD-L1 inhibitors, has achieved breakthroughs in advanced cancers, but its effectiveness is limited in early-stage tumors (such as colorectal cancer).
I. Cancer Immune Evasion: A Biological Conundrum of a "Cat and Mouse Game"
The immune system is the body's core defense against tumors, but cancer cells often use "immune evasion" mechanisms to disguise themselves, avoiding detection and attack by immune cells. While immune checkpoint therapy, represented by PD-1/PD-L1 inhibitors, has made breakthroughs in advanced cancers, its effectiveness is limited in early-stage tumors (such as colorectal cancer). Scientists have been searching for how tumors "blind" the immune system at an early stage. A study published in Nature revealed a key answer—the transcription factor SOX17. This molecule has been confirmed as the core "manipulator" of immune evasion in early colorectal cancer, providing a new perspective for cancer immunotherapy.
II. SOX17: A "Multi-tasker" from Embryonic Development to Cancer
SOX17 belongs to the SOX (SRY-related HMG-box) transcription factor family and plays an important role in embryonic development, angiogenesis, and organogenesis. Previous studies have found that SOX17 is abnormally highly expressed in various solid tumors (such as liver cancer and gastric cancer), but its function in the tumor immune microenvironment remains unclear.
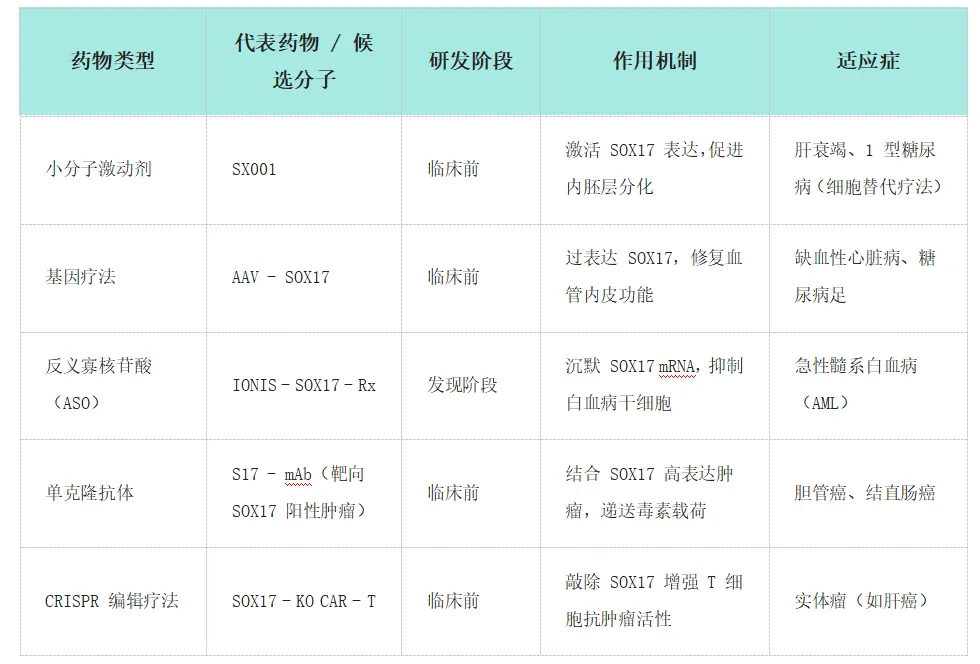
Research Drugs and Classification:
III. The Key Role of SOX17-Mediated Immune Evasion Mechanisms in the Early Progression of Colorectal Cancer
(I) Tumor-Specific Induction of SOX17 and Its Functional Verification
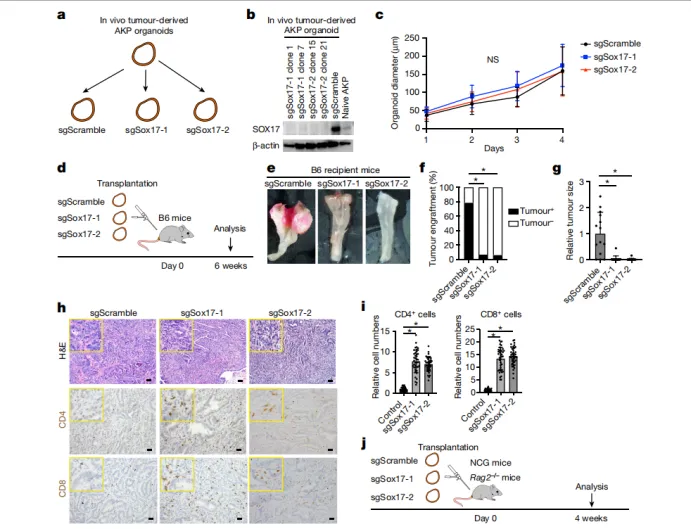
1. Through a colorectal cancer organoid in situ transplantation model, it was found that the in vivo microenvironment drives SOX17 expression
• Transcriptome and chromatin accessibility analysis showed that SOX17 is specifically upregulated in in vivo tumors but not expressed in in vitro cultured organoids.
• Immunohistochemistry confirmed that SOX17 is nuclear positive in transplanted tumors and spontaneous Apc-null adenomas, but not expressed in normal intestinal epithelium.
• Mechanistically, in vivo immune pressure (such as T cells) is not a necessary condition for SOX17 induction, suggesting that tumor cell-autonomous microenvironmental signals drive its expression.

(Image from the reference)
2. SOX17 deficiency leads to immune-dependent growth inhibition
• CRISPR knockout of SOX17 in AKP organoids showed normal proliferation in vitro, but the tumorigenesis rate decreased to 6% (vs 80% in the control group) after in situ transplantation in immunocompetent mice.
• In immunodeficient mice (NCG/Rag2 - /⁻), SOX17 deficiency does not affect tumorigenesis, indicating that its function depends on immune evasion.
(Image from the reference)
(II) Molecular Mechanisms of SOX17 Regulation of the Immune Microenvironment
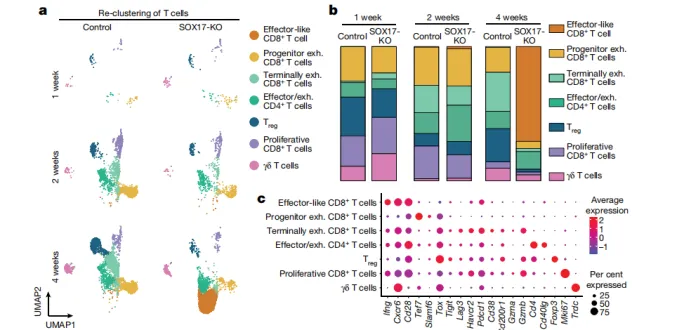
1. Inhibition of the IFNγ signaling pathway:
• Cell sequencing showed that the proportion of effector CD8⁺T cells (IFNγ⁺TNF⁺) was significantly increased in SOX17-deficient tumors, while the control group was mainly composed of exhausted T cells.
(Image from the reference)
• SOX17 directly binds to the Ifngr1 promoter (CUT&RUN verification), inhibits IFNGR1 expression, leading to reduced MHC-I expression and decreased CXCL10 secretion.
• IFNγ neutralizing antibodies or IFNGR1 knockout can reverse the immune clearance of SOX17-deficient tumors.
2. Driving fetal intestinal programs and tumor heterogeneity
• SOX17 activates fetal intestinal epithelial gene characteristics, promoting the differentiation of LGR5⁺ tumor stem cells to LGR5⁻, and LGR5⁻ cells have lower MHC-I expression and have an immune evasion advantage.
• In vivo lineage tracing showed that SOX17 deficiency increased the proportion of LGR5⁺ cells. 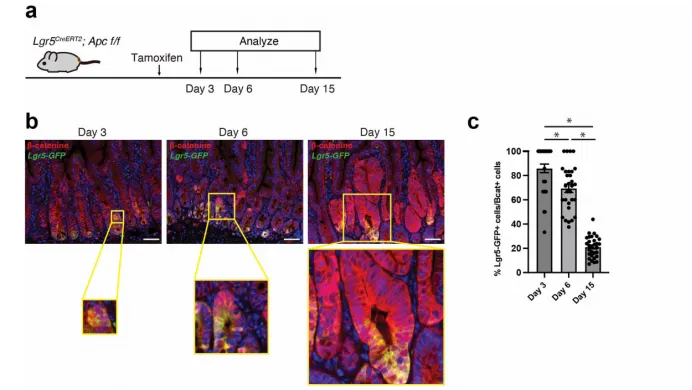
(Image from the reference)
In short: SOX17 is like putting an "invisibility cloak" on cancer cells, escaping immune killing in two ways:
• Turn off the alarm system: Inhibiting interferon-gamma receptors prevents immune cells from recognizing cancer cells (similar to cutting off the fire alarm);
• Disguise as normal cells: Activate the fetal intestinal development program, allowing cancer cells to blend into the normal cell population;
IV. Clinical Relevance and Therapeutic Implications
1. Human sample verification:
• 19 cases of adenomas and pT1 colorectal cancer showed high expression of SOX17, and were negatively correlated with CD8⁺T cell infiltration.
• Clinical significance: In the future, SOX17 can be detected in stool/blood to achieve earlier detection of precancerous lesions;
2. Potential for targeted intervention
• Inducible shRNA knockdown of SOX17 can inhibit the growth of established tumors.
• Therapeutic outlook: SOX17 inhibitors under development may enhance the efficacy of existing PD-1 inhibitors;
Scientists say: "SOX17 is like the 'invisibility switch' of cancer cells. Our findings provide a key target for early intervention. In the future, it may be possible to identify high-risk individuals through genetic testing and block this pathway before cancer develops." —Professor Omer H. Yilmaz
References: Goto N, Westcott PMK, Goto S, Imada S, Taylor MS, Eng G, Braverman J, Deshpande V, Jacks T, Agudo J, Yilmaz ÖH. SOX17 enables immune evasion of early colorectal adenomas and cancers. Nature. 2024 Mar;627(8004):636-645. doi: 10.1038/s41586-024-07135-3. Epub 2024 Feb 28. PMID: 38418875.
Note: This article is reproduced and shared for non-commercial purposes. Please contact us for removal if there is any infringement.
Keywords:
Reference Drug | Antibody Drug | Instrument | Reagent



