26
2025
-
02
Xiwen | Constream Biotech participation in the 6th ADC Drug Development Conference 2025 concludes successfully
The 6th ADC Drug Development Conference 2025, held on February 20th and 21st, was successfully concluded to great anticipation. Meticulously organized by the host, the conference attracted numerous industry experts, corporate representatives, and researchers. The event brought together cutting-edge perspectives and innovative technologies, creating a vibrant atmosphere over the two days and providing a high-quality platform for communication and collaboration in the ADC drug development field.
February 20-21, The 6th ADC Drug Development Conference 2025 was successfully held amidst great anticipation. Carefully prepared by the organizers, the conference attracted numerous industry authorities, corporate representatives, and researchers. The conference brought together many cutting-edge perspectives and innovative technologies. The atmosphere was enthusiastic throughout the two-day event, providing an excellent platform for communication and collaboration in the ADC drug development field.
Forum Highlights: Focusing on the Cutting Edge
This ADC drug development conference featured multiple themed forums covering key areas such as ADC drug target research, drug design, and clinical trials. Discussions centered around hot topics such as "New technology platforms/Transforming the design and development of ADC drugs: specific antibodies, stable linkers, safe and effective small molecule toxins; the significant role of translational medicine, biomarkers, and companion diagnostics research in the early stages to clinical trials of ADCs," etc. Many authoritative speakers from the industry were invited. They engaged in in-depth discussions and insightful presentations on the hot topics and challenges in ADC drug development. From the latest target discovery to innovative drug conjugation technologies, from sharing clinical application experiences to prospects for future development trends, each presentation provided attendees with new inspiration and insights, effectively promoting technological exchange and innovative breakthroughs in the ADC drug development field.

Popular Booths: Attracting Much Attention
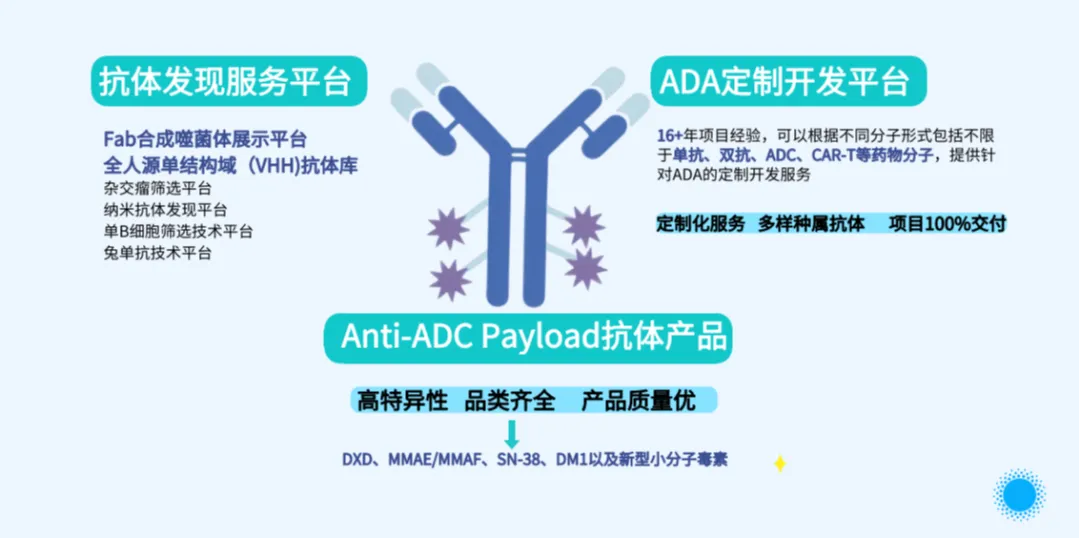
During the exhibition, Constream Biotech's booth became a focal point. With its advanced technologies and high-quality products in ADC drug development, it attracted numerous industry colleagues, experts, scholars, and potential partners. Many senior industry leaders and authoritative experts visited Constream Biotech's booth to learn more about our core technologies and products, including the fully human Fab synthetic phage library, fully human single-domain (VHH) antibody library, and Anti-ADC Payload antibody products. They spoke highly of Constream Biotech's technological strength and innovative achievements and engaged in comprehensive and multi-layered communication with the on-site staff: from in-depth analysis of technical principles to problem-solving in practical applications; from prospects for industry development trends to exploring future cooperation possibilities.






In-depth Exchanges: Working Together
Dr. Tao Weihong, CEO of Constream Biotech, delivered a keynote speech: Fully Human Fab Synthetic Library and VHH Synthetic Library Phage Display Platform Empowering ADC Drug Antibody Discovery With his profound professional knowledge and rich practical experience, he clearly explained the technical principles, advantages, and key roles of the fully human Fab synthetic library and VHH synthetic library phage display platforms in ADC drug antibody discovery. He then participated in a roundtable discussion, engaging in in-depth discussions with authoritative experts on early-stage development issues, including:
*Challenges and solutions in overcoming the challenges of ADC prototypes in the early stages of ADC drug development?
*ADC drug molecular design and optimization strategies?
*ADC technology platform selection?
*Strategies for evaluating the efficacy and safety of ADC drugs in early development to improve clinical success rates.
The atmosphere was enthusiastic, and the academic atmosphere was strong. The sharing and exchanges greatly inspired and enlightened the attendees.


Keywords:
Reference Drug | Antibody Drug | Instrument | Reagent



