09
2025
-
01
Xi News | Synthetic Fab HuCAL GOLD library screening helps Fizeto monoclonal antibody to market
On January 8, 2025, Tianjing Bio's application for the marketing of its CD38 antibody injection, Fizetomab, was accepted by the NMPA. This milestone is undoubtedly a new ray of hope for the second-line treatment of multiple myeloma patients.
On January 8, 2025, the NMPA accepted the marketing application for the CD38 antibody injection, Fezetomab, developed by Tianjing Bio. This milestone brings new hope to patients with multiple myeloma undergoing second-line treatment.
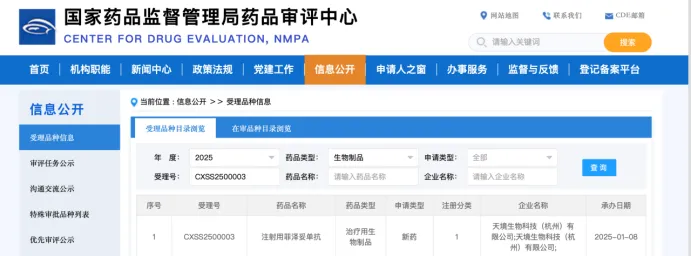
Figure 1. Acceptance of the BLA marketing application for Fezetomab injection
It is worth mentioning that Fezetomab was initially developed by Morphosys. In November 2018, Tianjing Bio introduced the Greater China rights to MOR202. Meanwhile, in terms of overseas rights, HI-Bio, with its accurate judgment of Fezetomab's potential, holds its overseas autoimmune rights. These rights became the focus of industry attention in May 2024, leading Biogen to decisively acquire HI-Bio for a staggering US$1.8 billion. This move strongly confirms the enormous future value of Fezetomab in the overseas autoimmune market and showcases the unlimited potential of this antibody molecule to the world.
However, the reason Fezetomab stands out among numerous antibody molecules and progresses to this crucial stage of marketing application is... its unique and highly advantageous screening method—Synthetic Fab HuCAL GOLD library screening ,起着至关重要的作用。
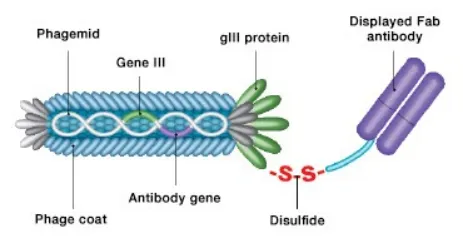
Figure 2. CysDisplay screening technology
The fully human synthetic library screening platform, based on advanced gene editing technology and precise molecular construction methods, can construct an extremely rich and high-quality fully human synthetic library, providing a solid foundation for screening antibody molecules with high specificity and high affinity. Its leading research on Fezetomab for the treatment of IgA nephropathy, AMR, and PMN autoimmune diseases has achieved positive progress and results.
At the 2024 American Society of Nephrology Kidney Week (ASN Kidney Week) international academic conference, research results showed a significant reduction in proteinuria in patients receiving Fezetomab treatment within a 6-month treatment period, with the therapeutic effect lasting more than 18 months after the last administration, while maintaining stable renal function. Fezetomab's ability to provide patients with lasting clinical benefits in lgAN treatment represents a significant breakthrough in this field.

Figure 3. Fezetomab KINDEY WEEK 2024
We continue to monitor the subsequent listing approval process of Fezetomab. We believe that with the advantages brought by fully human synthetic library screening, it will surely be successfully approved for listing, opening a new chapter that benefits patients and writes a legend in biomedicine. We also look forward to the fully human Fab synthetic phage library platform of Shanghai Constream Biotech helping more cooperative clients select Constream Biotech's fully human Fab synthetic phage library screening candidate antibody molecules for drug development, bringing clinical benefits and therapeutic value to more patients.
Keywords:
Reference Drug | Antibody Drug | Instrument | Reagent



